Physical Chemistry Questions Answers
Study physical chemistry in many universities and the board has been restructured with a greater emphasis on the conceptual and theoretical methodology and the applications of underlying basic concepts and principals. Naturally, the students find themselves unduly constrained when they are forced to refer to various books or online chemistry course materials to collect the necessary reading materials. It is primarily to help the students. This website provides systematic and comprehensive coverage of the theory as well as of the illustration of application and questions answers.
Ideal gas question
The molecules in the bulk of the container surrounded by other molecules in a symmetrical manner. This molecule on the whole experiences no net force of attraction. Now consider the molecule near the side of the container, which is about to strike one of its sides. These molecules contributing to the total pressure of the gas. This side molecule experiences a net force of attraction towards the center of the container. This results in decreasing the velocity of the molecule. Thus the molecule does not contribute much force as an ideal gas molecule where there been no forces of attraction. Thus the pressure of a real gas would be smaller than the corresponding pressure of the ideal gas.
Question:
What is the pressure of 2 moles of nitrogen gas occupying 10 lit volume at 270 C?Given, a = 1.4 atm lit2 mol-2 and b = 0.04 lit mol-1 using Van der Waals equation. What is the possible deviation from ideal gas law behavior?
Answer: Mole number = 2 mol a = 1.4 atm lit
2 mol
-2 b = 0.04 lit mol
-1 volume = 10 liter universal constant = 0.082 lit atm mol
-1 K
-1 temperature = (273 + 27)K = 300K. Therefore, pressure calculated from Van der Waals equation = 4.89 atm.
The pressure of the ideal gas, Pideal = nRT/V = (2 × 0.082 × 300)/10 = 4.92 atm. Thus,
Preal ㄑ Pideal due to the intermolecular attraction. The extent of deviation from ideal behavior = (4.92 - 4.89) = 0.300
Application of combined gas law
Question:
At 1 atm pressure and 300 K temperature, the volume of the gas 2000 cm3. What are the volume of this gas at 600 K temperature and 2 atm pressure?Answer: Combination of Boyle's and Charles's law, (P1V1)/T1 = (P2V2)/T2. Here, P1 = 1 atm, V1 = 2000 cm³, T1 = 300K, P2 = 2, T2 = 600K. Therefore, V2 = 2000 cm3
Question: One liter of a gas at 300 atm and 473 K compressed to a pressure of 600 atm and 273 K. The compressibility factors found from the curves are 1.072 and 1.375 respectively at the initial and final states. What is the final volume of the gas?
Answer:
Compressibility factor,Z = PV/nRT. Therefore, Z1 = P1V1/nRT1 and Z2 = P2V2/nRT2, where P1, V1, and T1 are the initial pressure, volume, and temperature and P2, V2 and T2 are the final pressure, volume, and temperature. Therefore, the final volume of the gas = 370.1 c.c.
How do you measure the mass of the gas?
Question
At 273 K and under the pressure of 100 atm the compressibility factor of oxygen 0.97. Calculate the mass of oxygen necessary to fill a gas cylinder of 108.5 lit capacities under the given conditions.
Answer
T = 273 K, Z = 0.97 and P = 100 atm.
Compressibility factor, Z = PV/RT where V is molar volume.
The molar volume of oxygen
Vm = ZRT/P
= (0.97 × 0.082 × 273 K)/(100)
= 2.17 lit mol-1
The mass of this molar volume will be equal to the molar mass of oxygen, which is 2.17 lit of oxygen equal to 32 gm.
Thus the mass of oxygen required to fill the gas cylinder 108.5 lit under the given condition
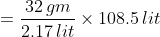
= 1600 gm
= 1.6 Kg
Question
Calculate the weight of oxygen necessary to fill up a cylinder of 5-liter capacity at 00 C and 100-atmosphere pressure when the compressibility factor 0.96.
Answer
Number of moles (n) = g/M gm mol-1 and Z = compressibility factor.
PV = Z × nRT or, n = PV/ZRT
∴ g/M = PV/ZRT or, g = PVM/ZRT
∴ Weight of oxygen = PVM/ZRT
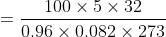
= 744 gm
Van der Waals question answer
For n mole real gas, Van der Waals equation of state \left&space;(&space;V-nb&space;\right&space;)=nRT})
^{2}}{\left&space;(unit\,&space;of\,&space;n&space;\right&space;)^{2}})
Unit of a = atm lit2 mol-2
CGS unit of a
= dyne cm-2 cm6 mol-2
= dyne cm4 mol-4
SI unit of a
= Newton m-2 m6 mol-2
= Newton m4 mol-2
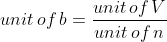
SI unit of b = m3 mol-1
CGS unit of b = cm3 mol-1
The dimension of a = [M L5 T-2 mol-1]
Dimension of b = [L3 mol-1]
Question
What is the Boyle temperature of nitrogen gas if a = 1.4 atm lit² mol⁻² and b=0.04 lit mol⁻¹?
Answer
Boyle temperature, TB = a/Rb
∴ Boyle temperature for nitrogen gas
= 1.4/(0.082 × 0.04) K
= 427 K


