Cobalt in Periodic Table
Cobalt (Co) is a silvery-gray ferromagnetic transition metal of Group 9 (VIIIB) of the periodic table element that is used in making alloys, and large quantities of its compounds. These compounds are used for making glass and ceramic materials.
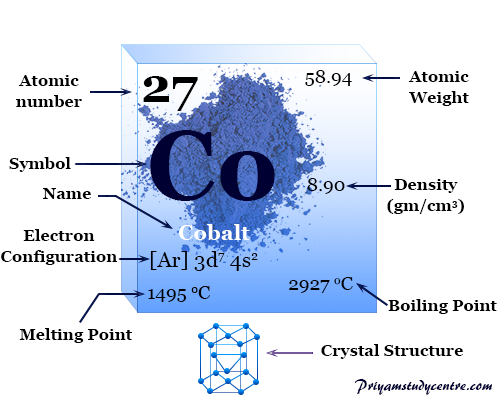
The hexagonal crystal lattice, cobalt has the chemical symbol Co, atomic number 27. Some physical properties of cobalt like melting point, boiling point, density, atomic weight, and electronic configuration of the metal are given below the picture.
Properties of Cobalt
- Cobalt is a lustrous silvery metal with a bluish tinge.
- The hardness and tensile strength of the metal are greater than steel.
- It is a ferromagnetic metal with a high curie temperature (1121°C).
- Finely divided metallic cobalt is pyrophoric like iron but compact metal is not attacked by air or water molecule at ordinary temperatures.
- The metal is less readily dissolved in mineral acids-like nitric acid and sulfuric acid.
- It does not attack by aqueous alkali but is readily dissolved in fused KOH at 550°C.
- The metal, cobalt reacts with halogens like fluorine, chlorine, bromine, and other non-metals like boron, carbon, phosphorus, and sulfur on heating.
Occurrence and uses
Cobalt is less common among the first transition metals except for scandium. It is the thirtieth most abundant element of all known elements that occurs mainly with nickel and arsenic. The principal minerals of cobalt are arsenides and sulfides like smaltite, CoAs2, erythrite, and Co3(AsO4)2.
Besides arsenic, sulfur, and iron, the minerals contain 4 to 10 percent of nickel, and a varying amount of silver, copper, and lead. South Africa and Canada are the main producers of cobalt and small reserves of metal are also present in Australia and Russian countries.
- Before the 19th century, large quantities of cobalt compounds are used in glass and ceramic industries but presently it is used in different fields like alloy or battery making and chemical catalysts.
- Cobalt compounds or blue pigments are used in glass and ceramic industries due to their artistic color.