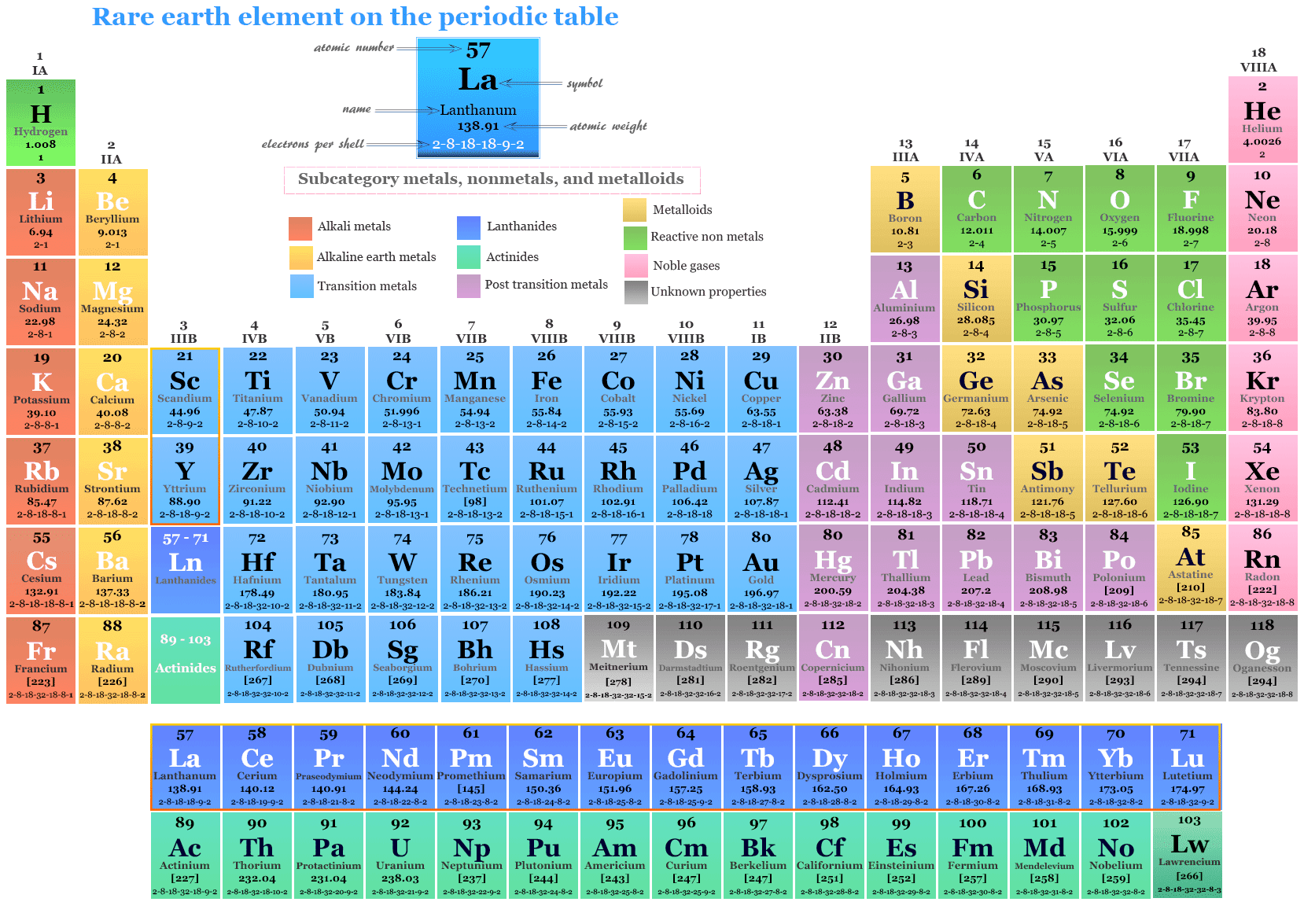
What are rare earth elements?
Rare earth elements or rare earth metals are the 17 elements such as scandium, yttrium, and 14 lanthanides elements of the periodic table uses widely in different fields of industries. Scandium and yttrium are part of d-block elements due to their outer electronic configuration. These two elements are considered rare earth metals because they occur in the same ore and the chemical properties of these elements are similar.
Image source: rare earth element
What are rare earth metals used for?
- The rare earth metals are widely used in metallothermic reactions due to their extraordinary reducing properties. These metals are also used as deoxidizing agents particularly in the manufacture of copper alloys.
- Alloys of lanthanides are called mish metals. The major constituents of mish metals are Ce (45 to 50%), La (25%), Nd (5%), and small quantities of other lanthanide metals with iron and calcium impurities. Mish metals are used for the production of different kinds of steel such as stainless and instrumental steel.
- Rare earth metals compounds such as CeO2, La2O3, Nd2O3, and Pr2O3 are widely used for decolourizing agents. Approximately one percent of CeO2 is used for the production of protective transparent glass blocks. These are widely used in nuclear technology.
- A compound such as CeS is used for the production of a special type of crucibles that is used for melting metals. Lanthanides oxides are used for polishing glasses.
- Rare earth compounds are widely used in paints, textiles, and leather industries.
- Salts of La, Ce, Eu and Sm are used as an activator of luminophores.
- In agriculture, dimals which are salicylates of Pr and Nd are used as germicides. Rare earth compounds are also used to manufacture insecticides, fungicides, and fertilizers.