Nitrogen in Periodic Table Elements
Nitrogen atomic number 7 and symbol N is the chemical element of Group 15 of the periodic table occurs as the diatomic gas dinitrogen N2 with no allotropic forms. The elements of this group like nitrogen, phosphorus, arsenic, antimony, and bismuth are collectively called pnictogen or pnicogen from the greek word choking. The element nitrogen and phosphorus are essential constituents of the living system used mainly for the manufacturing of fertilizer. The heavier member of the group, particularly arsenic is extremely toxic and causes water pollution.
Chemistry of Nitrogen
| Properties of Nitrogen | |
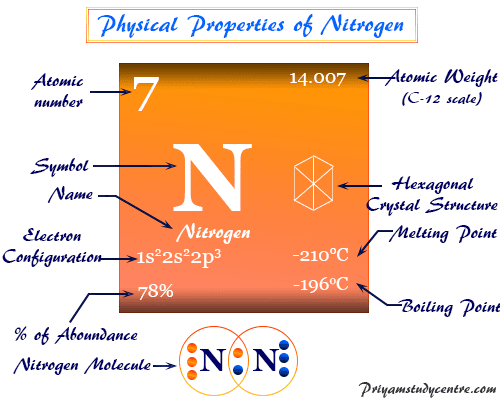
| Atomic Number | 7 |
| Atomic weight | 14.007 |
| Electronic Configuration | [He] 3s2 3p3 |
| Melting point | - 209.86 °C |
| Boiling point | - 195.79 °C |
| Density | 1.2506 g/l |
| Oxidation States | -3, -2, -1, +1, +2, +3, +4, +5 |
The chemistry of the element is very interesting. The remarkable tendency of catenation is observed in carbon and practically disappears in nitrogen. The importance of p-p pi-bonding increases with the increasing electronegativity of the atom. The electronic configuration of nitrogen is 3s2 3p3 with two paired electrons in the s-orbital and one unpaired electron in three p-orbitals. The electronic configuration suggests that nitrogen is closer to the next noble gas neon than the presiding noble gas helium. If we assuming the +5 cationic configuration by losing all the five outer electrons is just impossible. A huge amount of energy is required for this purpose. The sum of five ionization energy cannot be compensated by the gain of lattice energy by ionic bonding. Therefore nitrogen formed chemical compounds in +5 oxidation states are covalent compounds. The first ionization energy fall from nitrogen to bismuth is slow but metallic character along with the group steadily increases.
Nitrogen halides are restricted up to only the trihalides but phosphorus has pentahalides in addition to the trihalides due to the presence of vacant d-orbital in phosphorus atom. The d-orbitals of phosphorus can utilize to form pentavalent trigonal bipyramidal PCl5 with sp3d hybridization.
Uses
Nitrogen is the essential chemical constituent of plants and animals used widely to provide an inert atmosphere in metallurgy and in various chemical industries like the iron and steel industry or petrochemical industry. Liquid nitrogen is used as a refrigerant in low-temperature matching and grinding rubber or rubber-like substance, preservation of biological specimens.
Image Source and more about Nitrogen